Supramolecular self-assembly taking advantage of non-covalent and reversible intermolecular attractions allows the formation of hierarchical nanostructures. Recently, IAS research groups from Prof. Lee and Dr. Chan collaborated and they investigated the encapsulation and release mechanism of supramolecular self-assembly, which comprises of pentacene and 1,3,5-tris(4-carboxyphenyl)-benzenes at the octanoic acid/graphite interface (See Fig. 1) leading to a ground-breaking outcome. Such research outcome has been accepted for publication in the Journal of Physical Chemistry C, hosted by the American Chemical Society on 10th Dec 2019.
Upon using a scanning tunneling microscope, Lee’s group can observe the formation of supramolecular self-assembly. In particular, STM readily provides external stimuli including voltage bias and pulses, which can manipulate the supramolecular architectures on solid surfaces. STM results revealed that positive electrical pulses can promote the formation of such hybrids, whereas negative pulses selectively remove pentacene molecules from the complex. A non-equilibrium molecular dynamic has also been employed to securitize the detailed morphologies and the dynamics for such a process. Based on the experimental results obtained from Lee’s group, Chan’s group adopted effective multi-scale modeling in conjunction with the mean-field theory to calculate the nonbonded interactions between two molecules (see Fig. 2). He proved that under sufficiently large negative electric fields, both molecules can be separated completely (See Fig. 3). Most importantly, the theoretical and experiential results match, which validate the experimental results obtained by Lee’s group.
Essay Title: Electrical-pulse-induced Mixture and Separation in Surface Supramolecular Hybrids: STM Experiments and Theoretical Approaches
Journal: The Journal of Physical ChemistryC
Specific Information: Manuscript ID: jp-2019-10537k

Figure 1: Explore the generation and separation mechanism of supramolecular hybrid assembly.
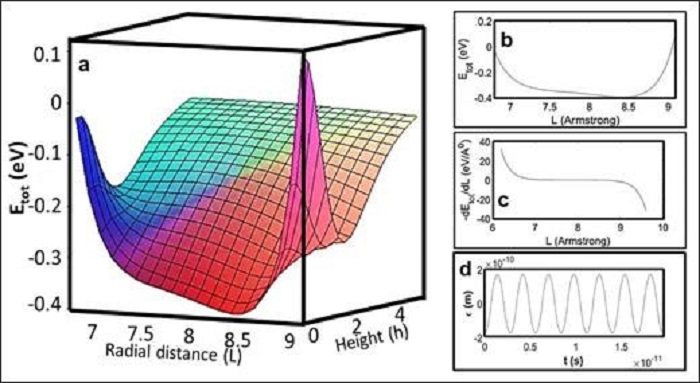
Figure 2: Interactions between two different non-covalently bonded substances, i.e. pentacene and BTBs.
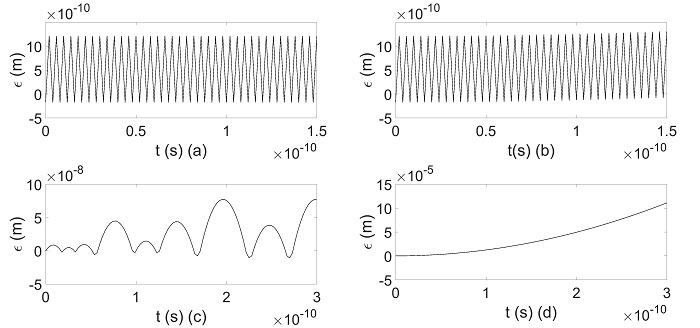
Figure 3: Dynamics of the pentacene when (a) Vb=0 V, (b) -1e-5 V, (c) -0.5 V and (d) -3.5 V.


