Recently, Professor Meng Li’s group from the Institute for Advanced Study of Shenzhen University published a paper titled ‘Coevolution of Eukaryote-like Vps4 and ESCRT-III Subunits in the Asgard Archaea’ in mBio (2019 IF=6.747), a top journal on the filed. This work provides evidences that the ESCRT complexes from Asgard archaea and eukaryotes are evolutionarily related and functionally similar. This paper was accomplished by Shenzhen University, Dalian Institute of Chemical Physics of Chinese Academy of Sciences and Shenzhen Institutes of Advanced Technology of Chinese Academy of Sciences. Professor Meng Li from IAS is the Co-corresponding author, and Postdoc. Zhongyi Lu from IAS is the first author.
Asgard archaea that is thought to include the closest prokaryotic relatives of eukaryotes, encodes many eukaryotic signature proteins, including homologues of ESCRT. Nonetheless, structural and functional features of Asgard ESCRT remain uncharacterized.
Here, we show that Vps4, Vps2/24/46, and Vps20/32/60, the core functional components of the Asgard ESCRT, coevolved eukaryote-like structural and functional features. Phylogenetic analysis and structural modelling show Asgard Vps4, Vps2/24/46, and Vps20/32/60 are closely related to their eukaryotic counterparts. Molecular dynamics simulation and biochemical assays indicate that there are eukaryotic-like interactions between Asgard Vps4 with Vps2/24/46 and Vps20/32/60. At last, The Asgard Vps4 partly, complements the vps4 null mutant of Saccharomyces cerevisiae, further supporting the functional similarity between the membrane remodeling machineries of Asgard archaea and eukaryotes.
This work provides evidences that the eukaryotic ESCRT seems to have been directly inherited from an Asgard ancestor, to become a key component of the emerging endomembrane system.
This work was supported by the grants from the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, the Basic and Applied Basic Research of Guangdong Province, and Shenzhen Sciences and Technology Program.
Paper link: https://doi.org/10.1128/mBio.00417-20.
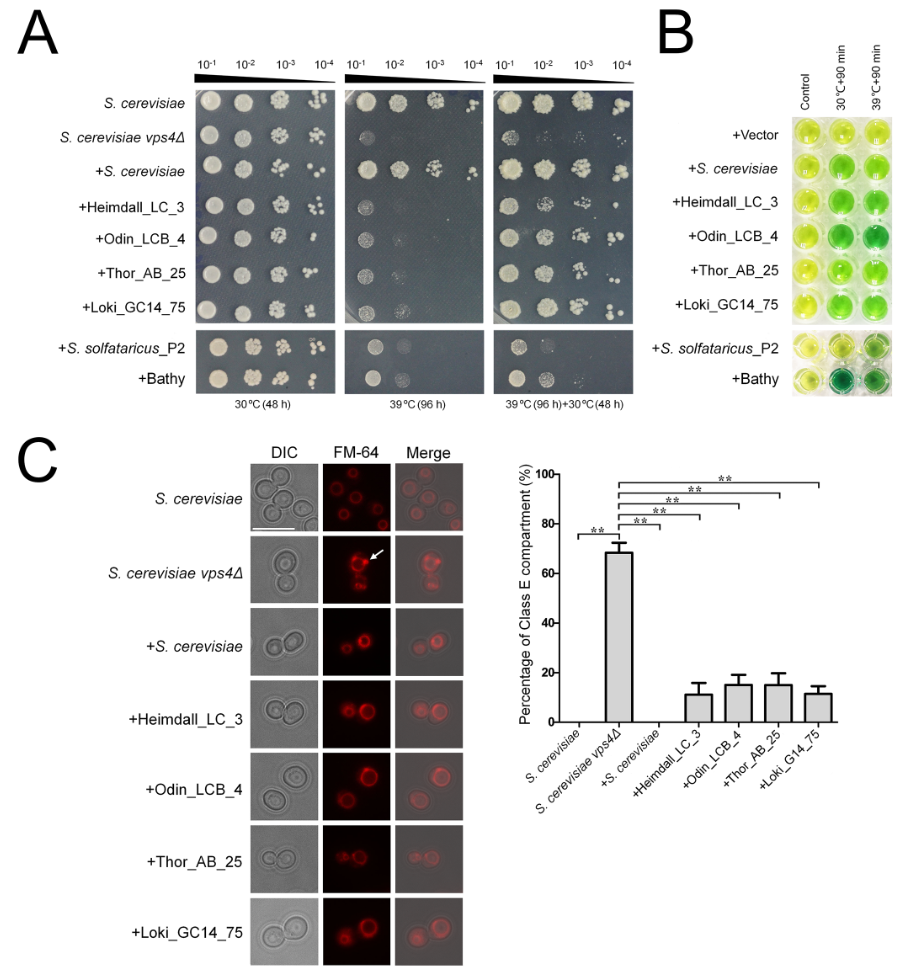
Figure. Functional complementation of Saccharomyces cerevisiae vps4 null mutants by Asgard Vps4


