Recently, Prof Shern-Long Lee of IAS at Shenzhen University together with Prof Gururaj kudur jayaprakash & Prof. E. Kumara Swamy (India) published an electrochemistry related paper in J. Mol. Liq. (IF: 5.0). The title is: Electrochemical and Quantum chemical studies of cetylpyridinum bromide modified carbon electrode interface for sensor applications.
In this work, both electrochemical experiments and quantum chemical simulations were performed to explore an important topic namely the cetyl pyridinium bromide (CPB) modified carbon paste electrode (CPBMCPE) employed for detection of dopamine (DA) and uric acid (UA). The electrode was found to display excellent electrocatalysis and increased surface area for simultaneously detecting DA and UA via decreasing their oxidation overpotentials when compared to the bare electrode, demonstrating high selectivity and sensitivity. Although surfactants can increase carbon-electrode sensitivity for DA detection, its mechanism remains elusive. Here, we used quantum chemical models to tackle the interface at which sensing (electron transfer) takes place. Our computational results reveal that the charged cationic head of CPBs can offer an additional electron transfer site at the CPBMCPE interface such that the sensing ability of CPE is comparatively improved after the CPB modification. Such electrodes were also applied for sensing UA in a urine sample, reaching a satisfactory of recovery up to 98%. To comprehend, we also modeled the power of electron-accepting and donating of CPB with other commonly used cationic surfactants.
Prof. Gururaj kudur Jayaprakash is the first author of this paper. Prof. Shern-Long Lee and Prof. E. Kumara Swamy are the corresponding authors. Prof. Xiuting Li is the co-author. The research was supported by NSFC general program (NSFC 21972095).
Paper link: https://www.sciencedirect.com/science/article/abs/pii/S0167732220301203
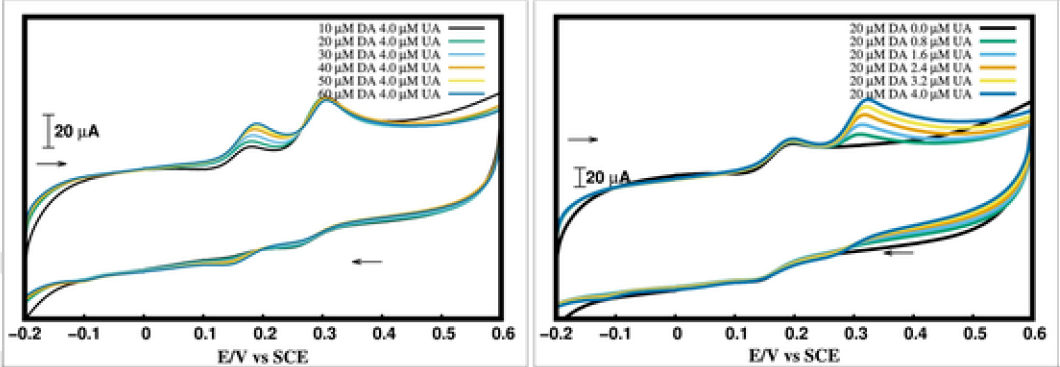
Fig 1: Cyclic voltammetry of the cetyl pyridinium bromide modified carbon paste electrodes for the simultaneous detection of dopamine and uric acid, demonstrating the high selectivity and sensitivity for such electrodes.
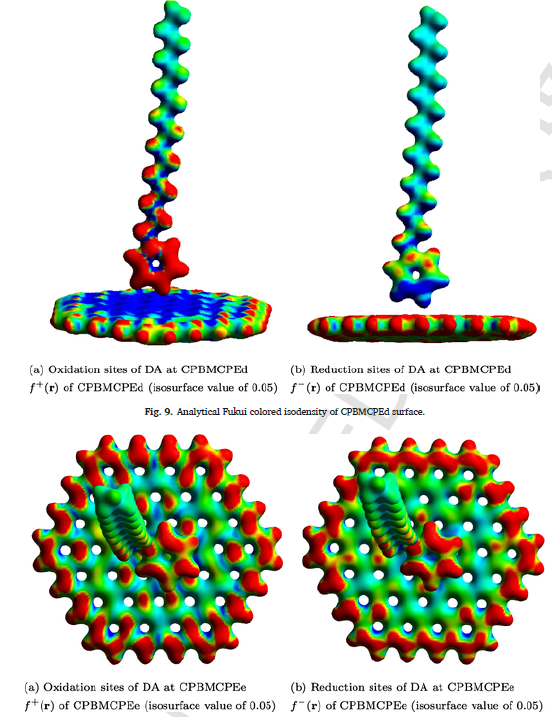
Fig 2: Using quantum chemical models (Fukui theory), we tackle the interface at which sensing and the electron transfer takes place.


