Xuan Zhao, Long Zhao, Qiuyun Xiao, Hai Xiong*
Intermolecular hydrogen-bond interaction to promote thermoreversible 2’-deoxyuridine-based AIE- organogels
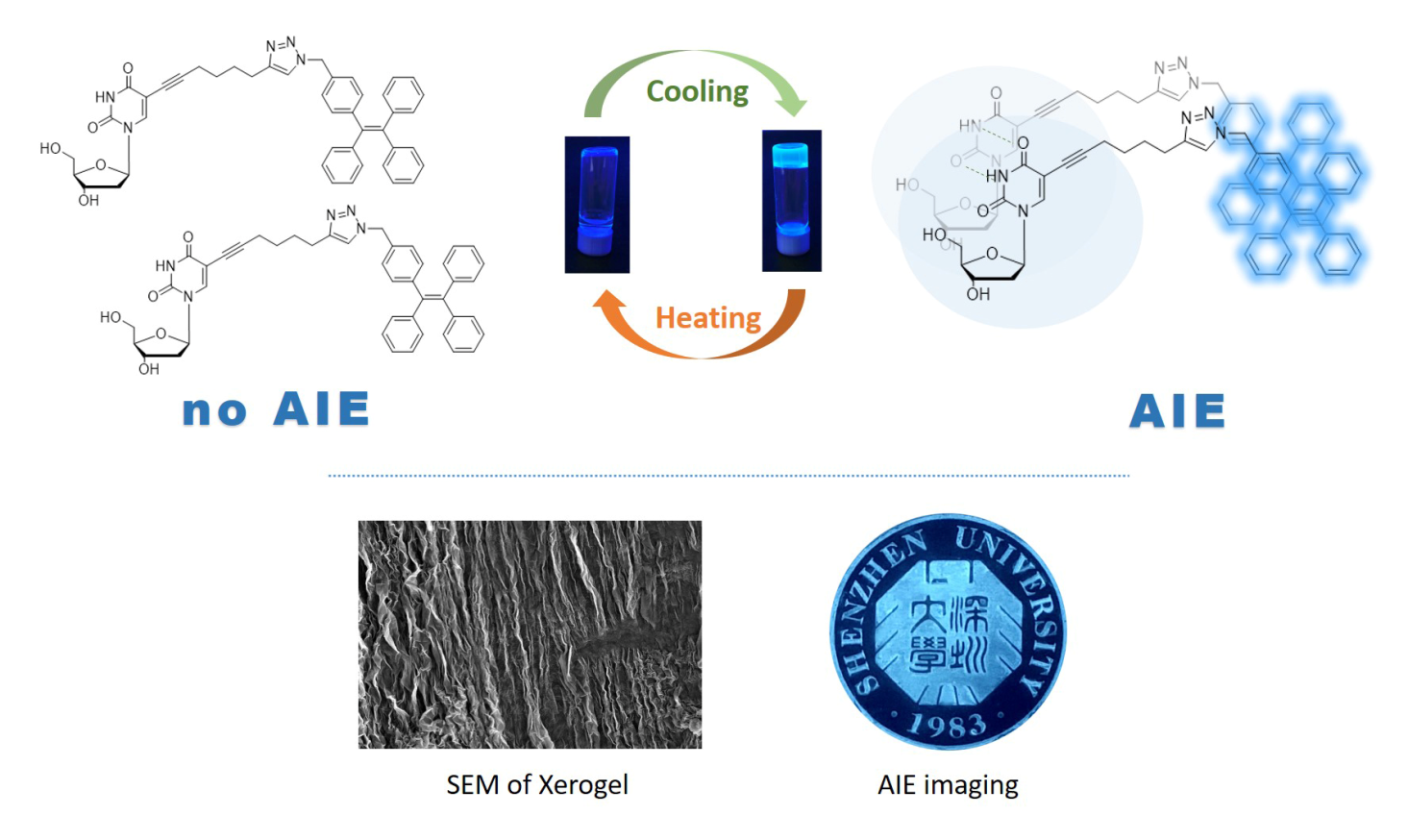
Fluorescent supramolecular nucleoside-based organogels or hydrogels have attracted increasing attention owing to their tunable stability, drug delivery, tissue engineering, and inherent biocompatibility for applications in designing sensors. As the temperature of a constant TPE-Octa-dU gelator at MGC as low as 0.2wt% was increased with gel to sol transition, a progressive decrease in the fluorescence intensity was observed. A 1H NMR study in [D6]Ethanol/H2O revealed the existence of intermolecular hydrogen-bond interaction between uridine nucleobase and triazole moieties. Based on these experiments, thus organogels induced by hydrogen bonding can promote an aggregation-induced emission(AIE) of TPE moiety. Thermoreversible gelation properties have been investigated systematically, including AIE-shapemorphing architecture owing to their unique solid-liquid interface and easy processability. At the same line, the related TPE-EdU derivative which was synthesized from 5-ethynyl-2’-deoxyuridine does not deliver organogels or hydrogels, and under similar circumstances TPE moiety of TPE-EdU does not efficiently exhibit AIE phenomenon either.
https://doi.org/10.1016/j.cclet.2020.10.008


