Recently, Prof. Xingke Cai from the Institute for Advanced Study at Shenzhen University published a research article in the Small, on December 12, 2023. The title of article is “Ternary Lithium Nickel Boride with 1D Rapid-Ion-Diffusion Channels as an Anode for Use in Lithium-Ion Batteries”. The first author of the paper is Mr. Wei Liu and Professor Xingke Cai is the corresponding author.
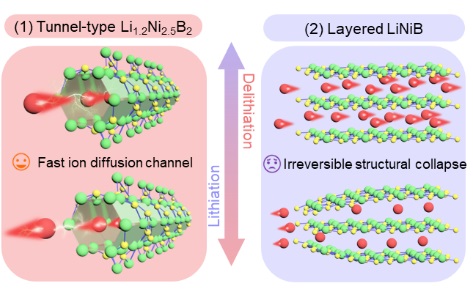
Figure 1. Illustration of crystal structure of tunnel-type Li1.2Ni2.5B2and layered LiNiB.
To date, various anodic materials have been commercialized, including Li metal, Si, graphite, and Li4Ti5O12(LTO). Li metal and Si display extremely high theoretical capacities but poor stabilities due to the growth of Li dendrites and considerable volume expansion. Graphite is a widely used anodic material with a relatively high theoretical capacity and cycle stability. However, its high diffusion barrier and Li plating close to the average charge potential lead to severe safety issues in practical applications. In addition, graphite also exhibits a poor performance at higher C-rates.Conversely, LTO exhibits an excellent cyclability and rate performance at high current densities, with a negligible change in volume during long-term insertion/extraction of Li+. Nevertheless, its relatively high working potential and low theoretical capacity limit its utilization.
Recently, transition metal borides attracted broad research interest as anodes for use in ion batteries owing to their good electrical conductivitiesand suitable working potentials. Boron is a semi-metal, and its metal compounds exhibit working potentials in the range of 0.6–1.0 V, which may avoid Li dendrite growth without losing much energy density.
Recent studies demonstrated that 1D tunnel-type materials display highly stable structures and excellent ion diffusion through their channels. Nevertheless, fluorides and oxides exhibit high redox potentials, which lead to low energy densities for practical applications as anodes. If transition metal borides may be used to fabricate tunnel-type structures, they should exhibit suitable working potentials and excellent cycling stabilities as anode materials for use in LIBs.
In this work, we studied a ternary alkali metal boride, Li1.2Ni2.5B2, which displayed a high Li+storage capacity and remarkable electrochemical stability and an excellent rate performance. In contrast to conventional transition metal borides, the introduction of Li atoms facilitated the formation of 1D Ni/B-based honeycomb channels during synthesis. This Ni/B framework successfully sustained the strain during Li+intercalation and deintercalation, and thus,the optimizedLi1.2Ni2.5B2anode exhibited an excellent cycle stability over 500 charge/discharge cycles. This electrode also exhibited superior reversible capacities of 350, 183, and 80 mA h g−1at 0.1, 1, and 5 A g–1, respectively, indicating the considerable potential of the 1D Ni/B framework as a commerciallyavailable fast-charging LIB anode.
This work was supported by the Natural Science Foundation of China, Guangdong Basic and Applied Basic Research Foundation and Science and Technology Innovation Commission of Shenzhen.
Link to the original article:https://doi.org/10.1002/smll.202309918


