On 15.1.2024, Professor Fang worked with the research team from University College London (UCL) together to publish a research paper entitled "Behaviour of alkali-activated fly ash-slag paste at elevated temperatures: An experimental study " in Cement and Concrete Composites (Q1, IF: 10.5). This paper studies the reaction process, microstructure evolution and mechanical properties development of alkali-activated fly ash-slag materials under high temperature. Professor Fang from Shenzhen University and Professor Zhang from UCL are co-corresponding authors.
Portland cement is the most widely used building binder material globally, but the production process emits a large amount of carbon dioxide, which is detrimental to the sustainable development of the ecological environment. The use of industrial solid waste to prepare new types of alkali-activated materials is considered a potential sustainable low-carbon material. Under high temperature conditions, alkali-activated cementitious materials undergo a series of chemical reactions, changes in microstructure, and the development of micromechanics, thereby affecting overall performance. To address this issue, this paper adopts XCT in-situ tracking test methods to systematically explore the interaction between chemical reactions, microstructure, and micromechanics of fly ash-slag based alkali-activated materials (AAFS) under high-temperature conditions. Results indicate that the compressive strength of AAFS paste rises by 77.5 % at 200 °C, followed by a mitigation from 200 to 600 °C and a regain at 800 °C. Different phases in AAFS paste including unreacted particles, reaction products and pores took up 30 %, 67.7 % and 2.31 % at ambient temperature, and 4.95 %, 84.8 % and 10.3 % after exposure to 800 °C, respectively, suggesting the recrystallisation with the formation of nepheline and gehlenite.
This study was supported by the National Natural Science Foundation of China and the Shenzhen Municipal Science and Innovation Commission. The original article can be found at:https://www.sciencedirect.com/science/article/pii/S0958946524000106#abs0010
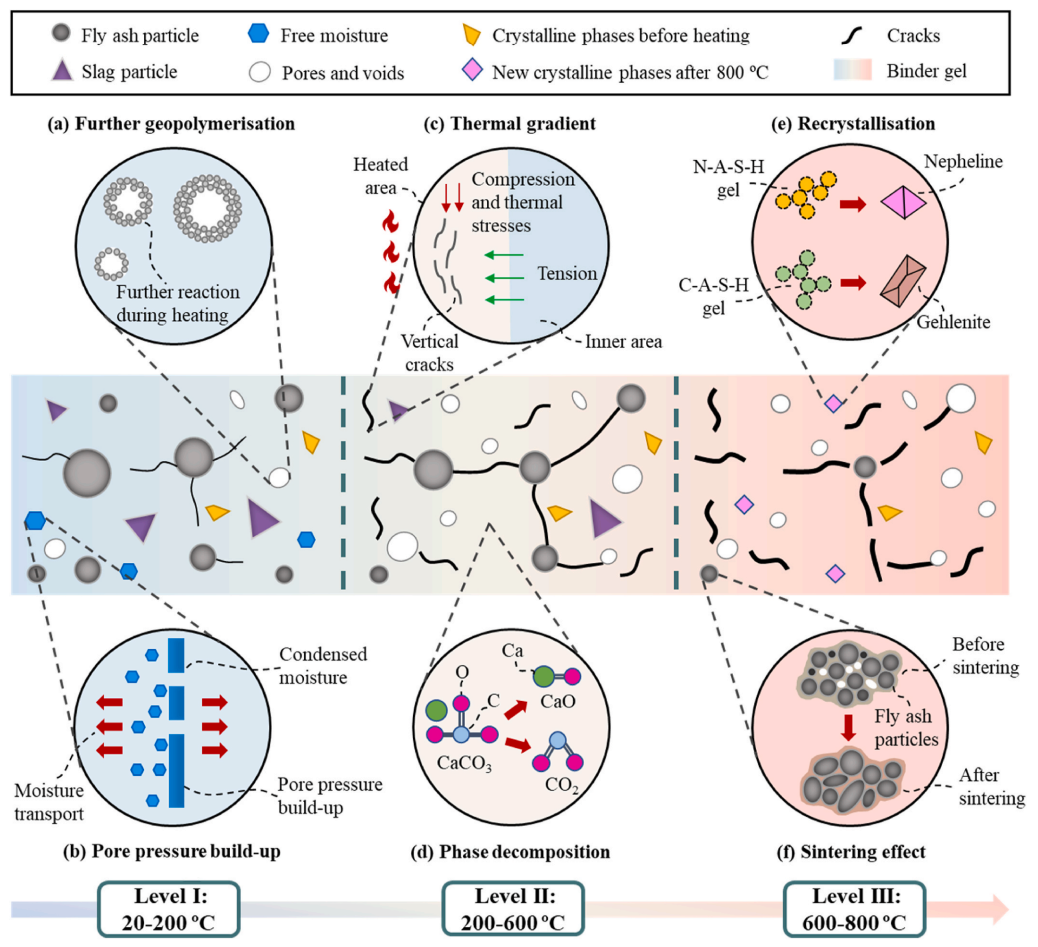
Fig.1 The degradation mechanism of alkali-activated materials at elevated temperatures


