On October 21, 2024, the research team led by Researcher Xingke Cai at the Advanced Institute of Shenzhen University, in collaboration with Professor Francesco Ciucci from the University of Bayreuth, published their findings titled "Out-of-plane coordination of iridium single atoms with organic molecules and cobalt-iron hydroxides to boost oxygen evolution reaction" in the journal “Nature Nanotechnology”. This paper reports a novel coordination structure involving the vertical coordination of single atoms with small-molecule carriers, differing from the traditional method where single atoms directly coordinate with atoms in the support, allowing for precise control over the catalytic site activity. The first affiliation is the Advanced Institute of Shenzhen University, with corresponding authors being Researcher Xingke Cai and Professor Francesco Ciucci; the first author is Jie Zhao, a postdoctoral researcher at Shenzhen University who has since moved on to continue his postdoctoral research at City University of Hong Kong.
Utilizing renewable energy for water electrolysis to produce hydrogen is a widely applied and mature technology, with the oxygen evolution reaction (OER) occurring at the anode being one of the main bottlenecks in this process. OER involves a four-electron transfer, making it a relatively slow reaction. Methods for loading noble metal single atoms onto metal hydroxide/oxide supports have been shown to effectively tune catalyst performance and are expected to further reduce overpotentials. It is generally believed that the coordination environment of these single atoms is limited to the atoms in the nearest neighboring support, which restricts the performance of single atoms to that of the metal hydroxide/oxide support, ultimately leading to performance limits.
In their study, the team discovered that when cobalt ions and MI molecules were used to construct Co-MOF and then implanted into a glycol solution containing other transition metal ions (such as iron or nickel), the MI would gradually dissolve, causing the entire material to recrystallize into low crystallinity cobalt-iron or cobalt-nickel hydroxides. Researchers introduced noble metal single atoms into these low-crystallinity hydroxides through this method and found that due to the significant size difference between noble metal atoms and iron/nickel atoms, the former would form dangling bonds at edge positions, directly connecting to MI molecules that lie out of the support plane. Compared to the atomic coordination environment formed by direct deposition of iridium atoms on the hydroxide surface, this direct coordination of iridium with MI small molecules not only exhibited reduced OER overpotential but also demonstrated good stability. The resulting Ir single atom catalyst, Ir/(Co,Fe)-OH/MI, provides an ultra-low overpotential of 179 mV at a current density of 10 mA cm−2, and 257 mV at a current density of 600 mA cm−2, alongside a remarkably low Tafel slope of 24 mV dec−1. Moreover, the overall mass activity of Ir/(Co,Fe)-OH/MI is 58.4 times that of IrO2. First-principles studies indicate that this exceptional OER performance can be attributed to the unique coordination between the Ir single atoms and MI molecules, which effectively redistributes charge around the Ir single atom site, leading to a favorable shift in the d-band center of the Ir and adjacent Co atoms and reducing the energy barrier of the OER reaction. This new form of coordination for catalytic sites offers more possibilities for tuning catalyst performance.
The work was supported by funding from the National Natural Science Foundation of China (No. 52003163, 22105129, and 22369020), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010670 and 2022A1515011048), Science and Technology Innovation Commission of Shenzhen (No. KQTD20170810105439418 and No. 20200812112006001). The Project was funded by China Postdoctoral Science Foundation (No. 2023M732352).
Link:https://doi.org/10.1038/s41565-024-01807-x
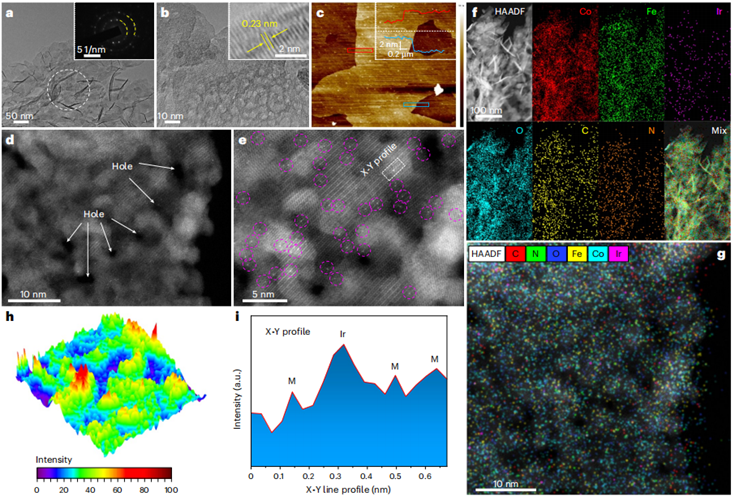
Fig. 1 Structural characterization of Ir/(Co,Fe)-OH/MI.


