On August 4, 2025, Prof. Wei Zhao’s team at Shenzhen University, in collaboration with Prof. Feng Zeng (Nanjing Tech University), Bin Wu (Nanyang Technological University, Singapore), Prof. Regina Palkovits (RWTH Aachen University, Germany) and Dr. Shulong Li (Chengdu University) published a research paper in Angewandte Chemie International Edition titled “Metallic Ni as Electron Acceptor Modulates the Redox of Catalytic Centers at Activated Ni0/Ni(OH)₂ Heterojunctions for Efficient Ethanol Electrooxidation.”
The study tackles the inefficiency of alkaline water electrolysis, where the sluggish oxygen evolution reaction (OER) consumes high energy. By replacing OER with the ethanol oxidation reaction (EOR), both energy demand can be lowered and valuable products like acetic acid generated. The team developed a Ni0/Ni(OH)₂ heterostructure electrode (ED-MHO@CP-a) via controlled electrodeposition. It delivered 573.7 mA·cm-2 at 1.37 V vs. RHE, 191 times higher than Ni(OH)₂, with acetic acid selectivity over 98% and a favorable revenue-to-cost ratio of 1.07. Mechanistic analysis revealed that Ni0 acts as an electron acceptor, promoting the Ni2+/Ni3+ cycle and lowering the energy barrier for intermediate formation, as supported by DFT calculations. A solar-driven EOR-assisted hydrogen production system based on this electrode achieved 14.4% solar-to-hydrogen efficiency, far exceeding conventional electrolyzers. This work offers a promising strategy for low-energy hydrogen production coupled with green chemical synthesis, advancing sustainable energy and catalysis.
Prof. Wei Zhao is the corresponding author. This work was supported by the Guangdong Basic and Applied Basic Research Foundation.
Paper link:https://onlinelibrary.wiley.com/doi/10.1002/anie.202510285
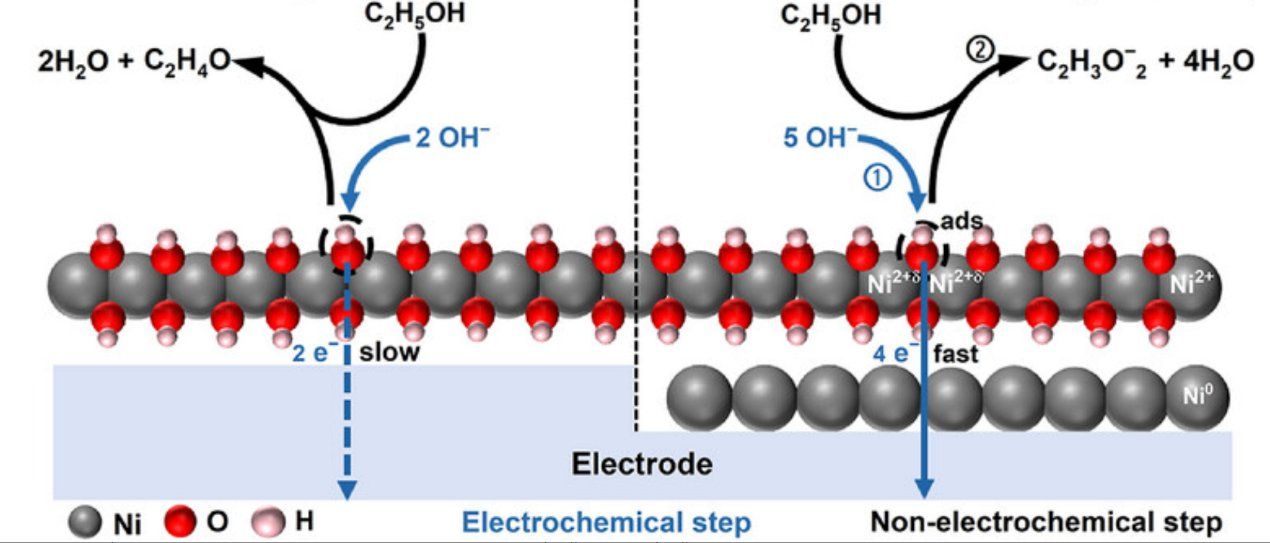
Figure 1. The Ni⁰/Ni²⁺ heterostructure formed through hydrophilic–hydrophobic surface regulation during the electrodeposition process enhances the Ni²⁺ ⇔ Ni³⁺ valence-state transition, lowers the kinetic barrier for acetaldehyde oxidation, and improves the efficiency of ethanol-to-acetate conversion


