On October 9, 2025, the team led by Professor Zang Shuangquan (Vice President for Academic Affairs at Zhengzhou University and recipient of the National Distinguished Young Scholars award) together with Researcher Wang Zheng from the Institute for Advanced Study, Shenzhen University, published a paper in the Journal of the American Chemical Society (JACS) entitled “Atomically Precise Copper Cluster for Highly Chemoselective Carbenoid Insertion into the N–H Bond of Aliphatic Amine.” Dr. Jia Teng is the first author; Professor Zang Shuangquan (Zhengzhou University) and Professor Wang Zheng (Shenzhen University) are co–corresponding authors. Shenzhen University is listed as the second contributing institution. In this work, Researcher Wang Zheng was responsible for density functional theory (DFT) calculations and mechanistic analysis.
Because aliphatic amines strongly coordinate to transition-metal catalysts (they are strong Lewis bases), this strong coordination typically suppresses catalytic activity and reduces chemoselectivity, posing a long-standing challenge for N–H insertion reactions. The research team designed and synthesized, for the first time, a highly stable copper cluster with dynamic catalytic sites (Cu₃NC⁽ᴺᴴᶜ⁾), which achieves unprecedentedly high chemoselectivity and catalytic efficiency in carbenoid insertion into aliphatic amine N–H bonds.
The study shows that the pyridyl groups on the Cu₃NC⁽ᴺᴴᶜ⁾ catalyst act as dynamic ligands that can competitively coordinate with the aliphatic amines, preventing catalyst deactivation by strong amine binding. The dynamically dissociating ligand also tunes the steric environment around the catalytic center, enabling highly selective monoalkylation of primary amines. The reaction proceeds via formation of a Cu₃NC⁽ᴺᴴᶜ⁾–carbenoid intermediate and achieves high chemoselectivity between C=C and N–H bonds. The turnover number (TON) for the N–H insertion reaches 79,200, far exceeding previous systems. Wang Zheng’s DFT calculations reveal that the Cu–carbenoid active center plays a key regulatory role along the reaction pathway, providing quantitative support for the experimentally observed high chemoselectivity.
This work not only demonstrates the unique potential of metal nanoclusters in organic catalysis, but also offers a new design strategy for efficient and stable catalysts based on a “dynamic ligand – rigid cluster core” cooperative approach. The reaction system is broadly applicable to late-stage functionalization and structural modification of pharmaceutical molecules—examples include clopidogrel, amoxapine, and vortioxetine.
The research was supported by the National Natural Science Foundation, Scientific and Technological Project of Yunnan Precious Metals Laboratory, and the Scientific Foundation for Youth Scholars of Shenzhen University.
Link: DOI: https://doi.org/10.1021/jacs.5c15400
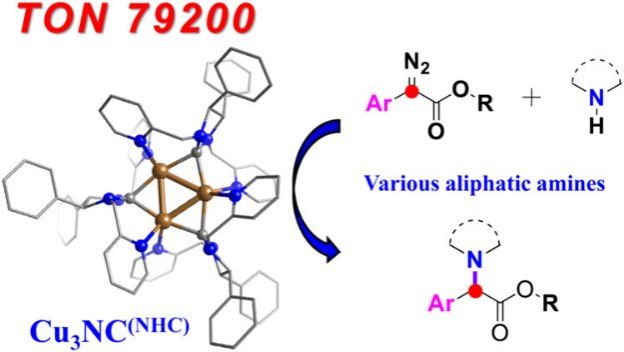
Figure 1. Atomically precise copper cluster achieves highly chemoselective carbenoid insertion into the N–H bond of aliphatic amines


