On 23.8.2023, Professor Fang worked with the research team from University College London (UCL) together to publish a research paper entitled "Multiscale study of microstructural evolution in alkali-activated fly ash-slag paste at elevated temperatures" in Cement and Concrete Composites (Q1, IF: 10.5). This paper studies the destruction mechanism and performance evolution of alkali-activated fly ash-slag materials under high temperature. Professor Fang from Shenzhen University and Professor Zhang from University College London are co-corresponding authors.
Alkali-activated materials (AAM) are recognised as promising alternatives to Portland cement (PC), as they not only lead to 60–80% less CO2emissions during manufacture which can boost sustainability of construction materials, but also possess superior thermal stability and high-temperature resistance. In recent years, a lot of attentions have been placed on the application of AAM as fire protection materials and thermal insulators. In recent years, the behaviour of AAM at elevated temperatures has been increasingly studied in terms of mechanical performance and thermal properties. However, there is lacking of research on the internal chemical reaction mechanism and microstructure evolution law of AAM under high temperature. In this study, multi-scale microstructure characterization and analysis method was used to systematically analyze the evolution mechanism of AAM at elevated temperatures. This study provides an in-depth insight into the damage mechanisms of AAM at elevated temperatures from a multiscale viewpoint, accounting for Level I: solid gel particles, Level II: gel matrix, and Level III: paste. Results indicate that the decomposition of C-A-S-H and N–C-A-S-H gels occurs while gel pores are filled at elevated temperatures up to 800 °C, along with the crack development, whereas micro-cracks are healed by melting and viscous sintering.
This study was supported by the National Natural Science Foundation of China and the Shenzhen Municipal Science and Innovation Commission. The original article can be found at:https://www.sciencedirect.com/science/article/pii/S0958946523003323
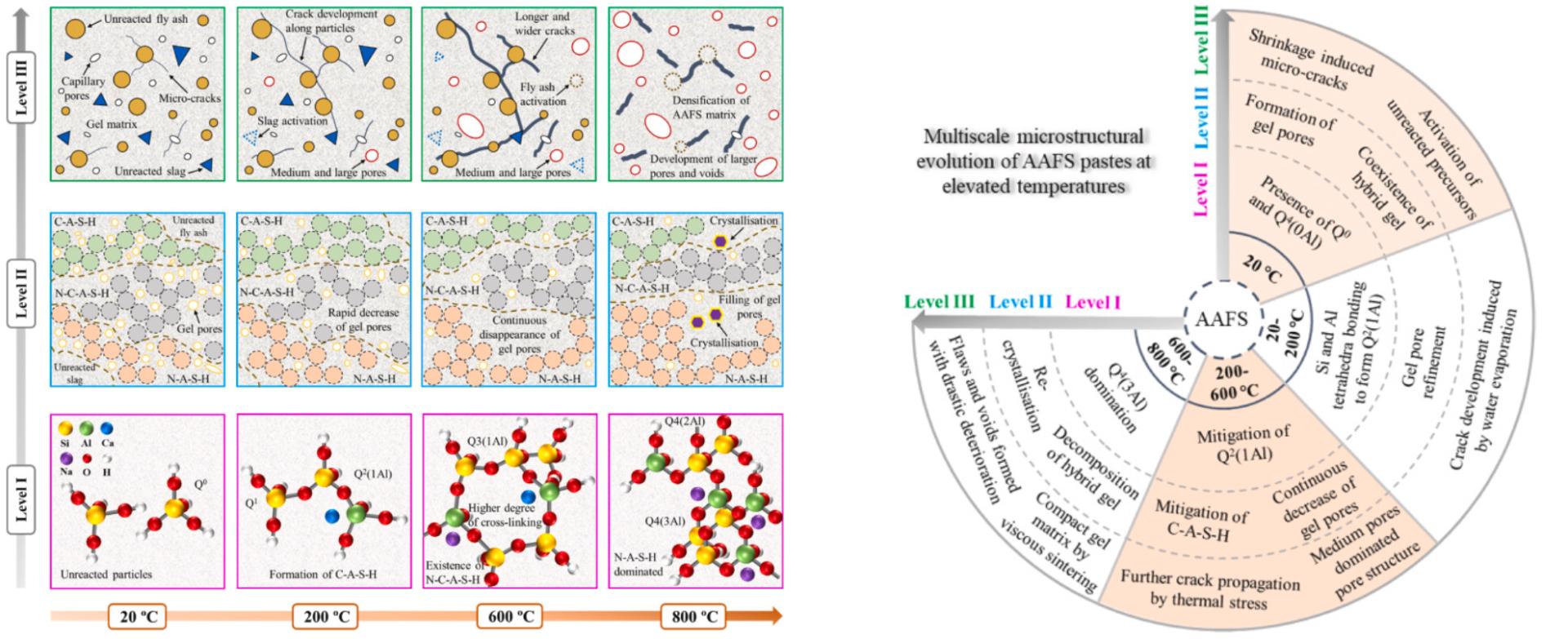
Fig.1 The multiscale microstructure evolution mechanism of alkali-activated materials at elevated temperatures


