Recently, the team from the Institute for Advanced Study, Shenzhen University, led by Researcher Cai Xingke, published an academic paper titled “Operando identification of the oxide path mechanism with different dual-active sites for acidic water oxidation” in Nature Communications. The first affiliation of the paper is Shenzhen University, with the first author being a postdoctoral researcher from Shenzhen University, Qianqian. Researcher Cai Xingke and Professor Yan Wensheng from the University of Science and Technology of China serve as corresponding authors.
In the paper, the research team constructed a Mn4-δ-O-Ru4+δ microstructure that has a locally symmetric structure but uneven charge distribution, achieving a reversal of the oxygen evolution reaction (OER) in an acidic electrolyte environment. Through a series of in situ characterizations, they verified that different types of bimetallic sites synergistically promote the occurrence of the electrochemical oxygen evolution reaction, allowing for manipulation of the OER pathway in principle.
The microscopic reaction pathway of the oxygen evolution reaction directly affects its performance, making it significant to reveal the structure-performance relationship between the microstructure of the catalyst and its macroscopic performance. Traditional adsorption oxygen evolution mechanisms (AEM) and lattice oxygen mechanisms (LOM) are limited by their inherent linear constraints and the characteristics of disrupted thermally stable structures, which restrict the balance between high activity and high stability. Recent studies have indicated a novel oxygen reaction mechanism (OPM), where oxygen atoms adsorbed on two adjacent catalytic sites couple directly to form oxygen molecules, thus achieving both high activity and high stability. Current reports on this mechanism are limited to the discovery of related catalysts and their performance studies, with little understanding of the underlying microscopic mechanism. It is commonly believed that doping with other elements can regulate the spacing of existing active atoms, allowing adjacent oxygen atoms to bond directly, with the doped atoms not acting as active sites in the reaction.
However, in this study, we found that when Mn atoms are substituted into the RuO2lattice, a unique Mn4-δ-O-Ru4+δ microstructure is formed. Based on in situ X-ray Fourier transform infrared spectroscopy, X-ray absorption spectroscopy, and differential near-edge spectroscopy, as well as isotopic labeling mass spectrometry, it was revealed that both Mn and Ru act as reactive active sites. The intermediates on the bimetallic active sites directly undergo the oxygen-oxygen coupling process, breaking the inherent understanding of the OPM mechanism in the scientific community. Since no lattice oxygen is produced in the reaction, its stability is also very good. Furthermore, because charge can be rapidly transferred within the Mn4-δ-O-Ru4+δ microstructure, its acid/oxidation resistance capability is far superior to that of RuO2.
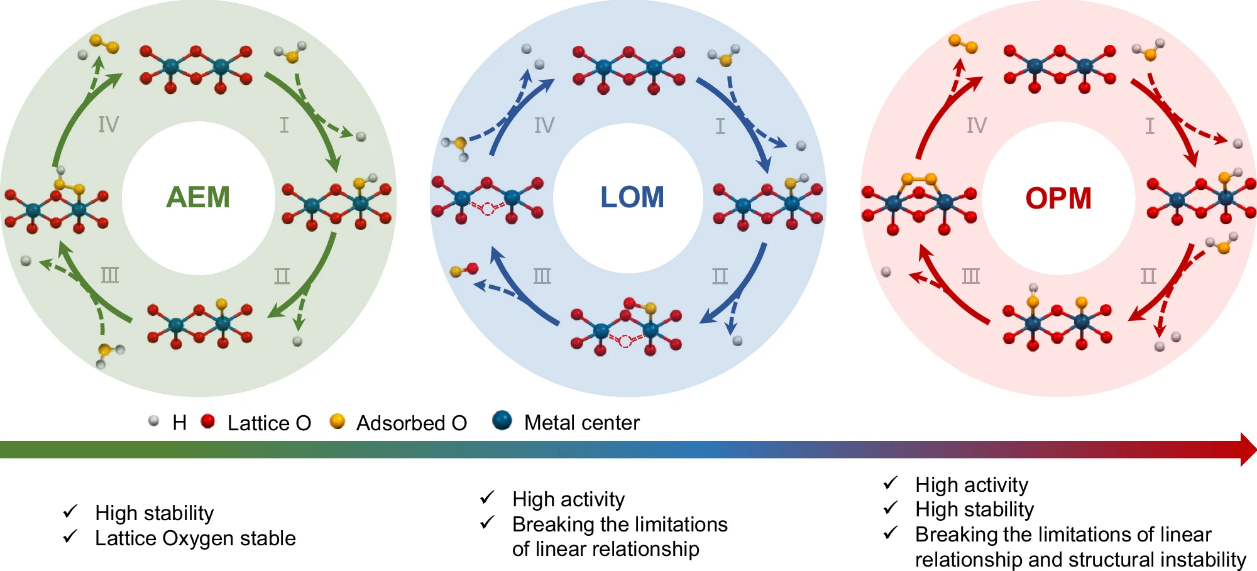
Figure 1. Schematic diagram of the AEM, LOM, and OPM reaction pathways and their characteristics.
The paper received support from the National Natural Science Foundation of China (52373266, 12305364, 12275271) and the National Key R&D Program of China (2023YFF0716100, 2021YFA1600800).
Paper link: https://www.nature.com/articles/s41467-024-52471-7


