In June 2025, the research team led by Dr. Lei Qin from the Institute for Advanced Study of Shenzhen University published a research paper titled "Regulating the coordination of solvent molecules with K salts for non-flammable and durable potassium-ion batteries with all-aluminum current collectors" in the Energy Materials and Devices. Focusing on the regulation of electrolyte solvation structures, this work innovatively applies fluorinated phosphate as a weakly solvating solvent in potassium-ion batteries (PIBs). By constructing an anion-rich solvation sheath, it achieves non-flammability and long-term operation of batteries with all-aluminum current collector systems. The Institute for Advanced Study of Shenzhen University is the first corresponding institution. The postgraduate student Yishuo Li is the first author, the undergraduate student Xinyang Zhang is the co-first author, and Dr. Lei Qin is the corresponding author of the article.
The full utilization of affordable PIBs based on all-aluminum current collectors is hindered by low specific energy, limited lifespan, and safety concerns, primarily due to the lack of suitable electrolytes for high-capacity electrodes. This paper introduces new molecular insights, from bulk solvation chemistry to interfacial behaviors, for designing compatible electrolytes. Fluorinated triethyl phosphate (FTEP) of tris(2,2,2-trifluoroethyl) phosphate was strategically selected as a low-polarity solvating solvent to create an anion-rich solvation sheath, albeit with reduced ion mobility at moderate concentration (1.0 mol·L-1). The deficiency of solvating-solvent molecules in the primary solvation sheath facilitates the formation of a protective layer derived from bis(fluorosulfonyl)imide anion decomposition, ultimately inhibiting undesirable side reactions at electrode/electrolyte interfaces. Moreover, FTEP as the sole solvating solvent endows the electrolyte with exceptional flame retardancy. The results provide crucial insights into the role of solvation chemistry on solvation structure and interfacial transport dynamics, critical for advancing the development of compatible electrolytes for high-performance PIBs.
The authors appreciate the funding support from the National Natural Science Foundation of China (Grant No. 52301280), the Postdoctoral HWYC Program, the Shenzhen Science and Technology Program (Grant No. JCYJ20240813142526034), the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2025A1515010810), and the Scientific Foundation for Youth Scholars of Shenzhen University (Grant No. 868-000001032171).
Original Article Link: https://doi.org/10.26599/EMD.2025.9370066
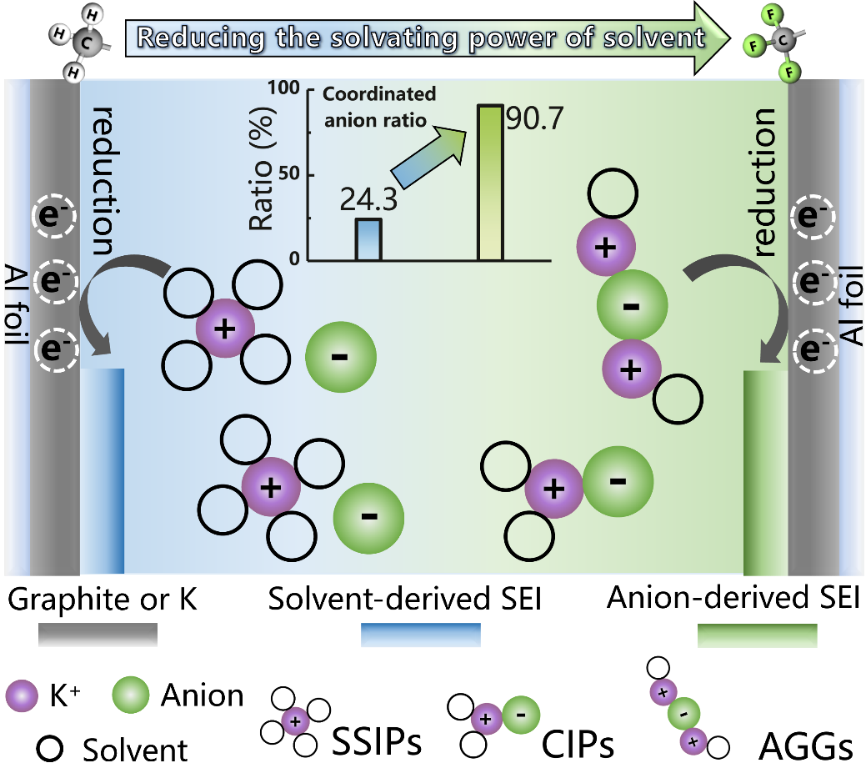
Figure 1 Graphic Abstract.


