On December 12, 2025, the team led by Wei Zhao from the Institute for Advanced Study at Shenzhen University systematically uncovered the mechanism of rhodium (Rh) and palladium (Pd) catalysts in the selective hydrogenation of biomass-derived furanic compounds through an innovative combination of density functional theory calculations and catalytic experiments. This achievement provides crucial theoretical support and practical guidance for the rational design of catalysts in the field of green chemistry.
As a renewable and carbon-neutral resource, the conversion and utilization of biomass is an important approach to reduce reliance on fossil fuels and mitigate climate change. 5-Hydroxymethylfurfural (HMF) and furfural (FF), as core platform molecules derived from biomass, can be converted into high-value products such as 2,5-bis(hydroxymethyl)furan (BHMF) and tetrahydrofurfuryl alcohol (THFA), which are widely used in renewable fuels, biodegradable plastics, pharmaceutical synthesis, and other fields. However, the selective hydrogenation pathways of these compounds are complex, and how to precisely regulate the reaction direction and improve the yield of target products has long been a major challenge in the industry.
To address this challenge, the Zhao Wei team selected representative noble metals Rh and Pd as model catalysts and conducted in-depth investigations into their adsorption preferences and reaction pathway differences toward HMF, FF, and their hydrogenated derivatives.
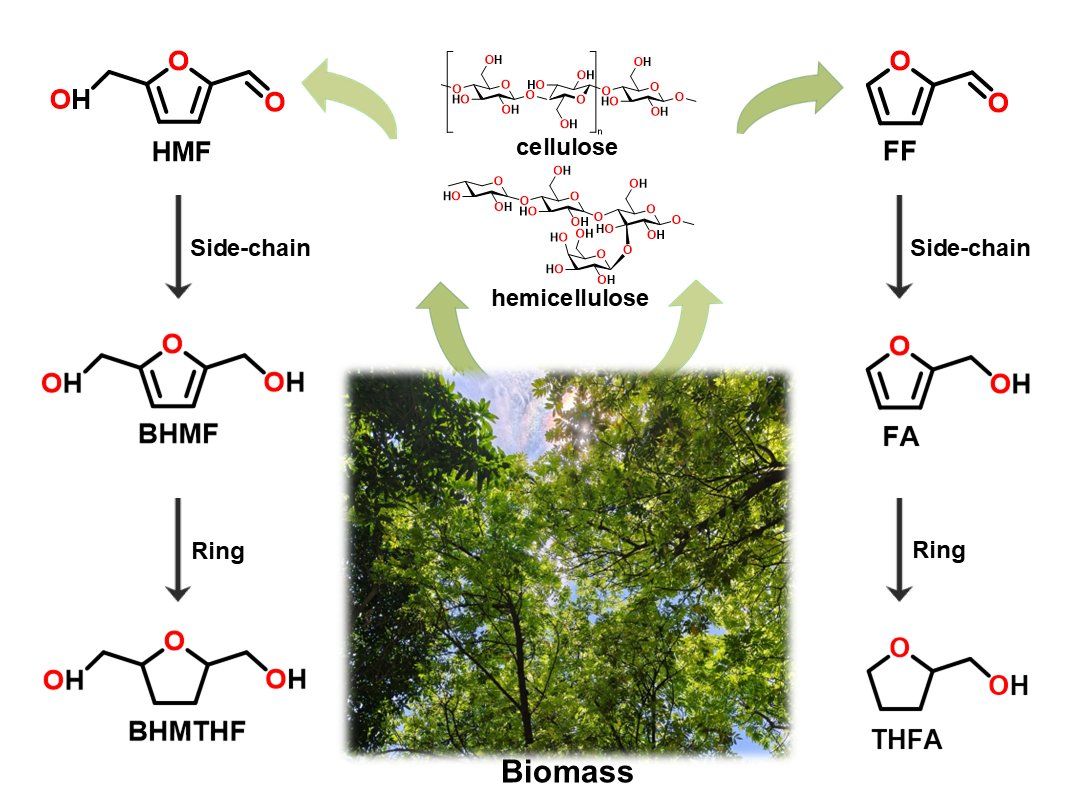
Figure 1: Pathways for the selective hydrogenation of HMF to BHMF and BHMTHF, and of FF to FA and THFA
The team carried out systematic catalytic experiments and in situ infrared reflection-absorption spectroscopy measurements. The study found that the two catalysts exhibit distinct catalytic selectivity due to differences in their electronic structures: the Pd catalyst shows excellent activity in the side-chain hydrogenation of HMF and FF, achieving a Faradaic efficiency of up to 65% for the conversion of HMF to BHMF; in contrast, the Rh catalyst is more proficient in catalyzing the ring hydrogenation of BHMF and FA, with Faradaic efficiencies of 32% and 35% for the formation of BHMTHF and THFA, respectively, which are significantly higher than those of the Pd catalyst.
The innovation of this research lies in the deep integration of theoretical calculations and experimental verification, clarifying the intrinsic law of electronic structure regulating catalytic selectivity. This achievement breaks the blindness of traditional catalyst development, provides precise guidance for the design of high-efficiency catalysts for specific biomass conversion reactions, and is expected to promote the industrialization process of converting biomass resources into high-value chemicals, contributing to the sustainable development of the green chemical industry.
The related research findings have been published in The Journal of Physical Chemistry C under the title “Mechanism Study for Metal-Catalyzed Hydrogenation of Biomass 5-Hydroxymethylfurfural and Furfural”. Professor Wei Zhao is the corresponding author, Min Hu (a 2022 undergraduate student) is the first author, and Shenzhen University is the independent communication institution.
Paper link:https://doi.org/10.1021/acs.jpcc.5c05856


