On 2025 December 23, Professor Meng Li’s group from the Institute for Advanced Study (IAS) of Shenzhen University published a paper titled Phylogenetic and functional characterization of Asgard primases in Molecular Biology and Evolution. Professor Meng Li is the co-corresponding author of the article. Dr. Zhimeng Li and Professor Yang Liu are the co-first authors. This work was completed jointly by the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Shenzhen University, and the Institute of Microbiology, Chinese Academy of Sciences (CAS).
The origin of eukaryotes is one of the central questions in the study of life's evolution and has long captivated the scientific community. The discovery of Asgardarchaeota has breathed new life into research on this topic. DNA replication machinery provides crucial molecular evidence of the link between archaea and eukaryotes, and the evolutionary trajectory of primase — composed of the catalytic subunit PriS and the non-catalytic subunit PriL — is key to solving this mystery. The research team found that Asgard archaeal primases can be divided into two distinct evolutionary lineages: the Heimdall and Loki groups (Figure 1). The Heimdall group exhibits greater similarity to eukaryotic primase, while the Loki group is more closely related to primases from non-Asgard archaea. This classification provides a clear framework for tracing the origin of eukaryotic primases. Structural analysis reveals that the C-terminal region of the Heimdall group's PriL subunit contains three consecutive α-helices, which can stabilize the iron-sulfur cluster. This structure is highly consistent with that of eukaryotic PriL, a feature absent in the Loki group and other archaea.
The research team successfully expressed and purified the primase heterologously from Candidatus Gerdarchaeota B18_G1 (a representative of the Heimdall group), which was isolated from deep-sea hydrothermal vent sediments, in Escherichia coli. Biochemical experiments revealed that this primase exhibits unique dual characteristics: it displays distinct archaeal primase features in terms of factors such as its substrates and divalent cation preferences, yet it synthesizes primers that are only 5–9 nucleotides (nt) in length. This short primer feature is characteristic of eukaryotic primases, whereas primases from other archaea usually synthesize much longer primers. The study also identified a motif that distinguishes archaeal and eukaryotic primases. Mutation experiments revealed that changes to the first amino acid residue of this motif significantly impact the enzyme's thermal stability and catalytic activity. Specifically, mutations towards the eukaryotic type reduced the enzyme's thermal stability by approximately 10°C, which is consistent with the evolutionary transition of eukaryotic ancestors from high-temperature to mesophilic environments.
By integrating phylogenetic, structural biology, and biochemical analyses, this work clearly delineates the evolutionary trajectory of primase from Asgard archaea to eukaryotes: the primase of Heimdall group archaea has acquired structural domains similar to those found in eukaryotes and developed a mechanism for synthesising short primers. This makes it a key evolutionary intermediate between archaea and eukaryotes.
The work was supported by National Natural Science Foundation of China, the Shenzhen Medical Research Fund, the PI Project of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) and Shenzhen University 2035 Program for Excellent Research.
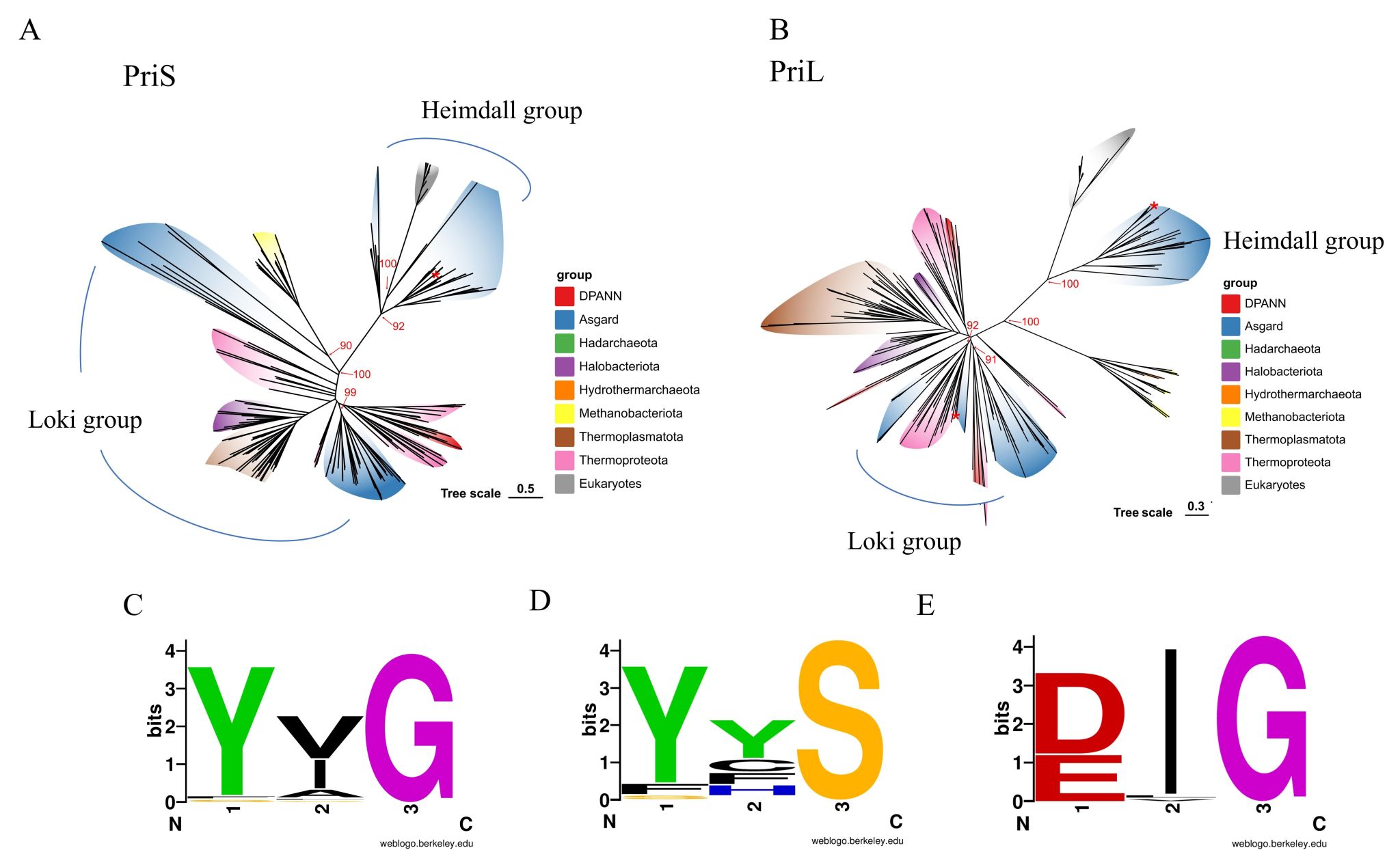
Figure 1. Phylogenetic analysis of primases from Archaea and Eukarya.
Original article link: https://doi.org/10.1093/molbev/msaf330


