On December 8, 2025, Prof. Wei Zhao’s research group at the Institute for Advanced Studies, Shenzhen University, in collaboration with Prof. Yongqiang Feng from Shaanxi University of Science and Technology, as well as other collaborators, published a research article entitled “High-Entropy Alloy Heterostructures with Tailored Interfacial Microenvironments for Efficient Electrocatalytic Water Splitting” in Small. The study reports a high-entropy alloy–RuNi heterostructured electrocatalyst with outstanding performance for alkaline water splitting and elucidates its interfacial catalytic mechanism. Prof. Wei Zhao is a co-corresponding author, and Shenzhen University is a co-participating institution.
Water electrolysis is a key technology for converting renewable electricity into green hydrogen, yet its practical deployment is limited by the sluggish kinetics of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which typically rely on expensive noble-metal catalysts. High-entropy alloys (HEAs), featuring multimetal synergy, lattice distortion, and high-entropy effects, offer a promising platform for tuning electronic structures and reaction intermediate adsorption. However, conventional HEAs often suffer from phase separation and elemental leaching under strongly alkaline conditions. To address these challenges, the researchers designed a CrFeCoNiRu–RuNi high-entropy alloy heterostructure (HEA–RuNi), composed of a CrFeCoNiRu HEA phase coupled with a RuNi phase. Through interfacial engineering, the catalyst enables precise regulation of the local reaction microenvironment while minimizing noble-metal usage, with a Ru content of only 1.78 wt.%. Electrochemical tests demonstrated excellent bifunctional activity in alkaline media. At 10 mA cm⁻², the HER and OER overpotentials were only 48 mV and 249 mV, respectively. Overall water splitting required just 1.69 V to reach 10 mA cm⁻², and the catalyst maintained stable operation for over 200 hours in an anion exchange membrane electrolyzer. Mechanistic studies combining in situ Raman spectroscopy, electrochemical impedance spectroscopy, and DFT calculations revealed that the heterointerfaces reconstruct the hydrogen-bond network of interfacial water, facilitating water dissociation and optimizing reaction intermediate adsorption. This work provides new insights into interfacial microenvironment engineering for the rational design of low–noble-metal, high-performance electrocatalysts for sustainable hydrogen production.
This research was supported by the Guangdong Basic and Applied Basic Research Foundation and related funding programs.
Paper link:https://doi.org/10.1002/smll.202510098
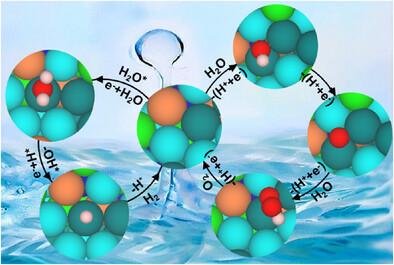
Figure 1. Optimized structure of the OER and HER intermediate structures on Ru active site at CrFeCoNi/RuNi interface.


